Today, the FDA is announcing revisions to the patient and provider fact sheets for the Moderna and Pfizer-BioNTech COVID-19 vaccines regarding the suggested increased risks of myocarditis and pericarditis following vaccination. For each vaccine, the Fact Sheet for Healthcare Providers Administering Vaccine has been revised to include a warning about myocarditis and pericarditis and the Fact Sheet for Recipients and Caregivers has been revised to include information about myocarditis and pericarditis. This update follows an extensive review of information and the discussion by CDC's Advisory Committee on Immunization Practices meeting on Wednesday.
The data presented at this meeting reinforced the FDA's decision to revise the fact sheets and further informed the specific revisions. The warning in the Fact Sheets for Healthcare Providers Administering Vaccines notes that reports of adverse events suggest increased risks of myocarditis and pericarditis, particularly following the second dose and with onset of symptoms within a few days after vaccination. Additionally, the Fact Sheets for Recipients and Caregivers for these vaccines note that vaccine recipients should seek medical attention right away if they have chest pain, shortness of breath, or feelings of having a fast-beating, fluttering, or pounding heart after vaccination.
The FDA and CDC are monitoring the reports, collecting more information, and will follow-up to assess longer-term outcomes over several months. As fatality rates due to COVID-19 markedly rise in those over 70 years of age, particularly in those with comorbidities, the advantages of vaccination in those with advanced age have been well appreciated during the pandemic. In contrast, as children and younger adolescents have had relatively lower rates of SARS-CoV-2–related symptomatic disease, or severe conditions requiring hospitalisation, intensive care, or death, there has been reticence and limited focus in vaccinations for these age groups.
This has been reinforced by a predominance of experience featuring asymptomatic or mainly mild, upper respiratory tract infection that resolves in a few days. Case rates with severe conditions and subsequent fatality in children have been low, though more common in adolescents with pre-existing severe life-threatening conditions . Presentation involves fever, elevated markers of inflammation and injury, circulatory shock, acute renal injury, cardiac dysfunction, coronary artery aneurysm, and myocarditis, requiring urgent intensive care support . As other trials currently in progress come to completion there will be greater confidence and emphasis on vaccination in children and adolescents, especially when vaccine production levels augment sufficiently to meet the international demand for all age groups. Coronavirus disease of 2019 (COVID-19), caused by severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2), has affected 169 million people across the globe and has resulted in more than 3.5 million deaths .
Two messenger RNA vaccines (Pfizer-BioNTech, Moderna) are now United States Food and Drug Administration -approved to reduce the risk and severity of COVID-19 infection . To date, 294.9 million COVID-19 vaccination doses have been administered in the US and the FDA recently expanded emergency use authorization of the Pfizer COVID-19 vaccine to include minors 12 years and older . On May 17, 2021, the US Centers for Disease Control and Prevention reported several cases of myocarditis within four days after receiving an mRNA-based COVID-19 vaccine, particularly in younger males after the second vaccine dose . A handful of subsequently published case series also suggests a possible association between acute myocarditis and mRNA-based COVID-19 vaccination in young adult males and in pediatric patients . Based on the evidence currently available, the CDC continues to recommend COVID-19 vaccination for everyone 12 years or older .
In this case series, we report the patient characteristics, cardiovascular magnetic resonance findings, and clinical course of 5 young male patients with acute myocarditis within 72 h after mRNA-based COVID-19 vaccination. Not surprisingly, highly publicised adverse events following immunisation with COVID-19 vaccine have been of great concern to the public and to health authorities world-wide, particularly when associated with the death of 'previously healthy' individuals. The US Centers for Disease Control and Prevention by mid-June 2021 had reported 1,226 cases of myocarditis after mRNA vaccination (Pfizer or Moderna, 29 December 2020–11 June 2021) from 296 million doses , an incidence of 4.1 per million doses. Consequently, CDC physicians and cardiologists evaluated 484 cases of patients aged under 30 years, with 323 individuals that strictly met definitions as per Dallas Criteria and Lake Louise Criteria for acute myocarditis, acute pericarditis or myopericarditis . The median age for these individuals was 19 years (range, 12–29 years) with first symptom onset at a median of 2 days (range, 0–40 days), and 92% with onset within 7 days.
Most patients were managed through mild clinical courses that resolved, with subsequent hospital discharge; there were no deaths. In contrast to adults, children have been largely spared COVID-19-related acute pulmonary infection and associated complications. However, children are vulnerable to a post exposure hyperinflammatory syndrome known as MIS-C. This syndrome can result in a severe clinical course requiring intensive care management. Ongoing surveillance of COVID-19 mRNA vaccines has identified potential post-vaccination adverse events. Recently, data presented to the Advisory Committee on Immunization Practices by the Centers for Disease Control and Prevention reported on incidence of myocarditis/pericarditis after approximately 300,000,000 COVID-19 mRNA doses in the United States.
Cases were much more common after the second dose, with a preponderance of men affected (approximately 5-10 times more frequent than in women). Similar to previous reports of myocarditis/pericarditis with other vaccines, one suspects that there is likely under-reporting of the true incidence on the basis of subclinical disease. The references for these cases series are included in Supplemental Appendix S1.
To date, myopericarditis has been reported to occur after both available mRNA vaccines (Moderna and Pfizer-BioNTech). The mRNA vaccines against COVID-19 infection have been effective in reducing the number of symptomatic cases worldwide. With widespread uptake, case series of vaccine-related myocarditis/pericarditis have been reported, particularly in adolescents and young adults. Men tend to be affected with greater frequency, and symptom onset is usually within 1 week after vaccination. On the basis of the available evidence, we highlight a clinical framework to guide providers on how to assess, investigate, diagnose, and report suspected and confirmed cases.
In any patient with highly suggestive symptoms temporally related to COVID-19 mRNA vaccination, standardized workup includes serum troponin measurement and polymerase chain reaction testing for COVID-19 infection, routine additional lab work, and a 12-lead electrocardiogram. Echocardiography is recommended as the imaging modality of choice for patients with unexplained troponin elevation and/or pathologic electrocardiogram changes. Cardiovascular specialist consultation and hospitalization should be considered on the basis of the results of standard investigations. Treatment is largely supportive, and myocarditis/pericarditis that is diagnosed according to defined clinical criteria should be reported to public health authorities in every jurisdiction. Finally, we recommend COVID-19 vaccination in all individuals in accordance with the Health Canada and National Advisory Committee on Immunization guidelines. In patients with suspected myocarditis/pericarditis after the first dose of an mRNA vaccine, deferral of a second dose is recommended until additional reports become available.
On its website, the Centers for Disease Control and Prevention explains that heart inflammation has been reported as a rare side effect of the Pfizer and Modern shots "especially in male adolescents and young adults," and more often after the second dose. When inflammation occurs, it usually happens within several days of vaccination. "Most patients with myocarditis or pericarditis who received care responded well to medicine and rest and felt better quickly," according to the CDC. Food and Drug Administration added a warning to patient and provider fact sheets for the Pfizer and Moderna COVID-19 vaccines. For each vaccine, the fact sheets were revised to include a warning about myocarditis and pericarditis after the second dose and with the onset of symptoms within a few days after receiving the shot.
A strong signal of myocarditis/pericarditis has been reported recently with mRNA COVID-19 vaccines in the United States . However, the US Advisory Committee on Immunization Practices has concluded that the benefits of mRNA COVID-19 vaccines continue to outweigh the risks of myocarditis and pericarditis even among young people. For persons over 30 years of age, the reporting rates were 2.4 and 1.0 per million second doses, respectively, for males and females. Additionally, pericarditis may be more common than myocarditis among older patients.
While cases of myocarditis and pericarditis linked to Covid vaccination are rare, they are likely underreported, the researchers said, pointing to a recent report by the CDC suggesting an incidence of about 4.8 cases of myocarditis per one million vaccinated individuals. "This study shows a similar pattern, although at higher incidence, suggesting vaccine adverse event underreporting," they wrote. Myocarditis and pericarditis are diseases that cause inflammation of the heart that can occur following infections or immune-related diseases. Symptoms of myocarditis and pericarditis can vary but may include shortness of breath, palpitations, irregular heartbeats, and chest pain. The public is advised to seek medical attention if they experience any of these symptoms following vaccination with Covid-19 vaccines. Consistent with previously published case series, all of our patients were males younger than 40 years.
Among these 323 patients, the median age was 19 years, 291 (90%) were males, and the median interval from vaccination to symptom onset was 2 days . From previous literature, it also appears that myocarditis typically occurs more commonly in males, and the incidence is highest amongst adolescent and young adults . On the other hand, estrogen may play a protective role in myocarditis by stimulating inhibitory T regulator cells and inhibiting proinflammatory T-cells . Clinicians should be aware of the risk of myocarditis and pericarditis with mRNA vaccines and those most likely to be affected.
They should be alert to presentations such as acute chest pain, shortness of breath and palpitations that may be suggestive of myocarditis after vaccination, especially in adolescent or young males. Coronary events are less likely to be the source of such symptoms among younger people. Results A small number of reports of myocarditis and pericarditis had been submitted to each database examined. These events are very rare according to reporting rates of spontaneous adverse reactions. The events were more frequently reported amongst males, and most reports came from vaccinees aged under 30 years. The typical clinical course of these events is mild, with full recovery in most cases.
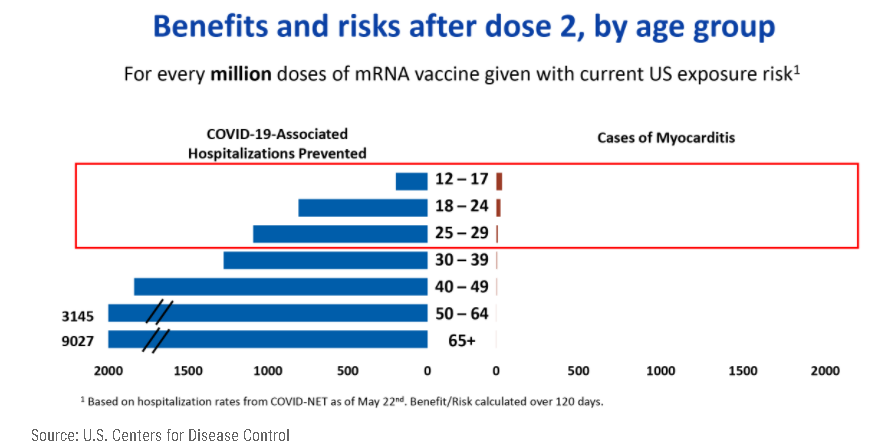
Reporting rates were 40.6 cases per million second doses of mRNA COVID-19 vaccines for males aged 12 to 29 years and 2.4 per million second doses for males aged 30 or older. For females their reported rates were 4.2 and 1.0 per million second doses in these age groups and the highest reporting rates were among males aged 12 to 17 and those aged 18 to 24 years. In Israel, 27 of the 148 cases occurred around receipt of the first vaccine dose and 121 occurred within 30 days after the second vaccine, with most cases in men aged 16 to 19 years. In their review of the Pfizer-BioNTech vaccine, regulators paid close attention to an American health care claims database, which found that the risk of the conditions in 16- and 17-year-old vaccinated boys could be as high as 1 in 5,000. The cases in the database were unconfirmed, the F.D.A. cautioned in an analysis published this week, but they were considered a reasonable estimate of the possible risk. Even in the worst-case scenarios of post-vaccination myocarditis and pericarditis modeled by the F.D.A., the benefits of vaccination still outweighed the risks, the analysis said.
Health Canada recently updated the label for the Pfizer and Moderna shots, warning of rare reports of myocarditis, but experts have continued to stress that the risk of heart inflammation is far higher from COVID-19 than from vaccines. One study by Case Western Reverse University researchers found the risk of myocarditis/pericarditis among male teens aged 12 to 17 diagnosed with COVID was nearly six times higher than their risk from a first or second dose of an mRNA vaccine. For girls aged 12 to 17, the heart risk was 21 times greater from a COVID infection. "If you're focused on heart inflammation, the safer bet is to take the vaccine," a Case Western Reserve researcher involved in the study told New Scientist. Even greater caution should be employed for administering these vaccines to individuals who experienced myocarditis after receiving a first dose.
For now, among such individuals second doses should be considered only in those with high risk of exposure to SARS-CoV-2, the virus that causes Covid-19, or high risk of complications if they develop the disease. In contrast, chest pain, irregular heartbeat, heart palpitations, shortness of breath and light-headedness could indicate myocarditis or pericarditis. Symptoms of these conditions have generally occurred within seven days of vaccination.
In the state of Rhode Island, a total of 693,578 people have received COVID-19 vaccination with at least one dose and a total of 635,432 people are fully vaccinated against COVID-19 infection . Nevertheless, continued clinical follow-up of these patients, perhaps with repeat CMR in 3–6 months, is needed to better understand whether there are any meaningful medium- and long-term effects of myocarditis following mRNA COVID-19 vaccination. Severity of myocarditis and pericarditis cases can vary, but "reports have increased since April, mostly in young males 16 and older, several days after vaccination, and more often after the second vaccine dose.
All cases of myocarditis and pericarditis following COVID-19 mRNA vaccination should be reported to public health authorities according to local/provincial/territorial reporting guidelines. Consider referral of the patient to a Special Immunization Clinic to discuss and advise on future COVID-19 vaccinations . People with a history of myocarditis or pericarditis unrelated to mRNA COVID-19 vaccination should consult their clinical team for individual considerations and recommendations. The National Advisory Committee on Immunization recommends deferral of the second COVID-19 vaccination for those with myocarditis/pericarditis after the first dose until more information is available . The retrospective study, which has not yet been peer reviewed, used the US vaccine adverse reporting system to identify the rate of post-vaccination myocarditis among and year olds between January and June 2021 after the second dose of the Pfizer-BioNTech vaccine. The researchers concluded that the rate of cardiac adverse events after the second dose exceeded the expected rate of 120 day covid-19 hospital admission at both a moderate and a high incidence of SARS-CoV-2 infection.
Background A signal of myocarditis and pericarditis following mRNA COVID-19 vaccines first emerged in Israel in May 2021. The reporting rates of these events indicate that they are very rare given the high numbers of vaccinated persons. Males and younger vaccinees appear more frequently affected, more often following the second vaccine dose. As vaccine programmes progress with the focus shifting to younger people, it is possible that more cases of myocarditis and pericarditis will be reported. In Australia, to 11 July 2021, the Department of Health Therapeutic Goods Administration has received 50 reports of suspected myocarditis and/or pericarditis out of 288 total adverse event reports after 3.7 million doses of Pfizer mRNA COVID-19 vaccine . A research letter published in JAMA involving 40 hospitals in Washington, Oregon, Montana and Los Angeles county and more than two million people who received at least one dose of a COVID vaccine estimated an incidence of one case of myocarditis in 100,000 vaccinations.
In another large study out of Israel published in the New England Journal of Medicine, researchers estimated that, for every 100,000 people who get the Pfizer vaccine, one to five would likely develop myocarditis. However, the risk of heart inflammation was 11 events for every 100,000 people infected with COVID. A 37-year-old male with no known health problems presented to the emergency department 2 days after receiving his second dose of the Pfizer COVID-19 vaccine with chest pain radiating to the left arm.
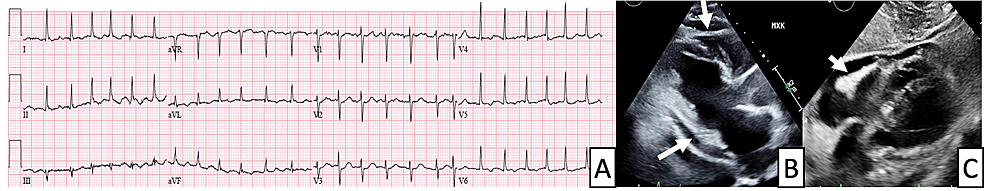
A 12-lead ECG showed ST elevations in the lateral leads and ST depression in lead V1. He was taken emergently to the cardiac catheterization laboratory where invasive coronary angiography was negative for obstructive coronary artery disease. On admission, serum troponin I was elevated at 26 pg/ml and the erythrocyte sedimentation rate was 32 mm/h (normal range 0–15 mm/h). SARS COVID-19 PCR testing, other viral serologies, and bacterial testing were all negative.
T2-weighted images showed no hyperintensity in any of the myocardial segments but T2 mapping showed elevated relaxation time, consistent with myocardial edema. The patient was diagnosed with acute myocarditis and was sent home without any medications with cardiology follow up. Messenger RNA coronavirus disease of 2019 (COVID-19) vaccine are known to cause minor side effects at the injection site and mild global systemic symptoms in first 24–48 h. Recently published case series have reported a possible association between acute myocarditis and COVID-19 vaccination, predominantly in young males. The benefits of vaccination in protecting against COVID-19 greatly outweigh the risks of adverse events including myocarditis.
The risk of myocarditis from COVID-19 infection is almost four times higher than from vaccination. An increased risk of heart inflammation has been observed in people who have received mRNA COVID-19 vaccines in overseas studies, particularly in males under 30 years of age after the second vaccine dose. More data have become available since the GACVS statement of 26 May 2021, with more countries reporting myocarditis and pericarditis in individuals who received COVID-19 mRNA vaccines. The reported cases have typically occurred within days of vaccination, more commonly among younger males and more often following the second dose the of COVID-19 mRNA vaccines. A common feature of these post-COVID-19 vaccine reports of myocarditis [, , , , , ] is the very low incidence, general similarity of patient demographics, condition, symptom onset and outcomes. These reports also highlight the importance of decision making as to when to include cardiac MRI in evidence not only in confirming each case but also in assisting with excluding confounding abnormalities.
As far as we are aware, there have been no previously published reports of this association, although Takotsubo cardiomyopathy has been reported after influenza vaccination . In this case, cardiac MRI provided valuable confirmative diagnostic support and reinforces the need to characterise specific cardiomyopathic features and subtype. As a 'stress'-associated cardiomyopathy, Takotsubo and its excess catecholamine-mediated myocardial stunning may provide targets for further investigation following post-COVID-19 vaccination systemic and cardiac inflammation and inform medical management of cardiac dysfunction.
The impact of the global health crisis due to the virus Severe Acute Respiratory Syndrome Coronavirus-2 (SARS-CoV-2)—the causative pathogen of the coronavirus disease 2019 (COVID-19)—has begun to alter with the timely development, approval and administration of vaccines . Although SARS-CoV-2 infection primarily targets the respiratory system [, , , , ], it is now recognised that the infection and its clinical manifestations are systemic [, , , , , ], and also affecting the cardiovascular system of adults and children [, , , , , , , ]. Cardiac complications of variable severity with acute and long-term sequelae are now known to include acute myocardial injury, arrhythmias, vasculitis and endothelial dysfunction, thrombosis, myocardial fibrosis, and myocarditis [, , , , , , , , , , ].
On the basis of our current state of knowledge, the association between myocarditis/pericarditis and COVID-19 mRNA vaccines in children and younger adults merits careful consideration for practitioners. Cases tend to be mild, do not usually require specific interventions, and potential risks of the vaccine are outweighed by the well defined risks of COVID-19 infection. A thoughtful approach to evaluation, management, and reporting of suspected cases of myocarditis/pericarditis is indicated as a key public health measure. The known and potential benefits of COVID-19 vaccination outweigh the known and potential risks, including the possible risks of myocarditis or pericarditis. Also, most patients with myocarditis and pericarditis who receive care responded well to medicine and rest and quickly felt better.
A separate analysis of data from the Vaccine Safety Datalink network of health care organizations looked at confirmed cases of myocarditis or pericarditis among people ages years within seven days after vaccination with either mRNA vaccine. Confirmed cases have occurred mostly in male adolescents and young adults aged 16 years or older. But given the hundreds of millions of vaccine doses administered, says the CDC, reports of myocarditis and pericarditis are rare.
As the country continues to push for more people to get vaccinated against COVID-19, some remain concerned over rare cases of heart inflammation—myocarditis and pericarditis—linked to the Pfizer-BioNTech and Moderna mRNA vaccines. While some parents may be thinking twice about teen vaccination, medical experts reassure that the risk of myocarditis and pericarditis are far lower than the risks of serious illness or death from contracting COVID-19. Although myocarditis and pericarditis have many virological and immunological causes, a causal link was suspected due to the immunological reaction to the mRNA COVID-19 vaccine. Individuals are recommended to rest and refrain from heavy strenuous activities for 1 week after mRNA COVID-19 vaccination, which will be helpful during the rare occurrence of myocarditis or pericarditis. Individuals experiencing chest pain, shortness of breath, or palpitations after receiving the mRNA vaccine are advised to seek immediate medical attention.





















No comments:
Post a Comment
Note: Only a member of this blog may post a comment.